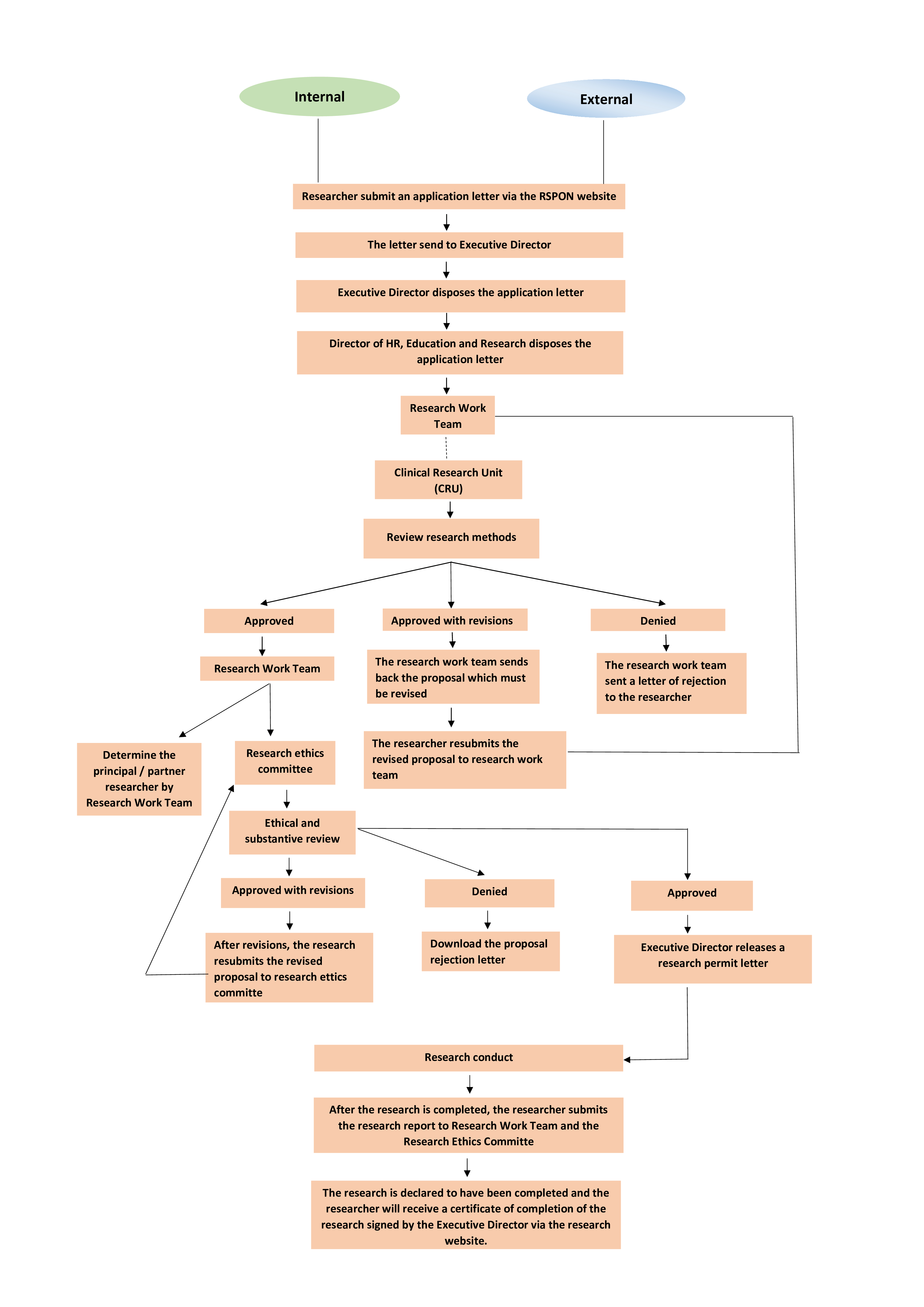
FLOW OF APPLICATION FOR ETHICAL REVIEW AND RESEARCH PERMIT
ALUR PERMOHONAN KAJI ETIK DAN PERIZINAN PENELITIAN
ALUR PERMOHONAN KAJI ETIK DAN PERIZINAN PENELITIAN

| No. | Nama Dokumen | Download |
|---|---|---|
| 1. | Ketentuan Umum Penelitian / General Consent of Research | General-Consent-Penelitian-Ketentuan-Umum-Penelitian_081240.docx |
| 2. | Ringkasan Penelitian / Research Summary Executive | Template-Executive-Summary-Penelitian_081314.docx |
| 3. | Rincian Anggaran Biaya / Budgeting Plan | Template-Rincian-Anggaran-Biaya-Penelitian_081334.docx |
| 4. | Formulir Skrining / Screening Form | Form-Skrining-Penelitian-Research-Screening-Form_153119.docx |
| 5. | Surat Permohonan Izin Penelitian / Research Permit Application Letter | |
| 6. | Proposal Penelitian / Research proposal | |
| 7. | Clinical Trial Protokol Outline* (For Clinical Trial Use Only) | Clinical-Trial-Protokol-Outline_V1_2025_152521.pdf |
Address : MT Haryono Street, Kav. 11, Cawang, East Jakarta, Indonesia, Postal Code 13630
+62 811-9003-4720 cru.nbch@rspon.co.id
RESEARCH by SIMRS. © All rights reserved